Electroplating
Plating

The history of plating is long, and the gold plating (amalgam process) of the “Great Buddha of Nara” is well known in Japan. So, why is plating necessary?
Plating has the following effects.
- Beautifies the appearance of materials
- Prevents rust and corrosion of materials (e.g., steel)
- Improves electrical conductivity
- Resistance to abrasion
- Improves sliding properties (slipperiness), etc.
Electroplating method (e.g., zinc plating)
Electroplating reacts as follows.
Positive pole (oxidation reaction)
-
Zn → Zn2++ 2e
(Dissolution of zinc)
-
2OH– → H2O + 2e + O2 ↑
(Oxygen gas generation)
Negative pole (reduction reaction)
-
Zn2++ 2e → Zn
(Plating deposition)
-
2H++ 2e → H2 ↑
(Hydrogen gas generation)
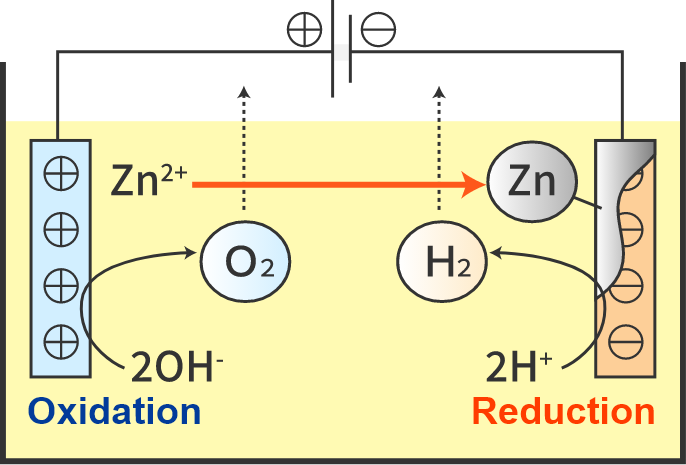 At this time, the negative electrode is galvanized.
At this time, the negative electrode is galvanized.
Why is steel galvanized?
Sacrificial corrosion protection for galvanization
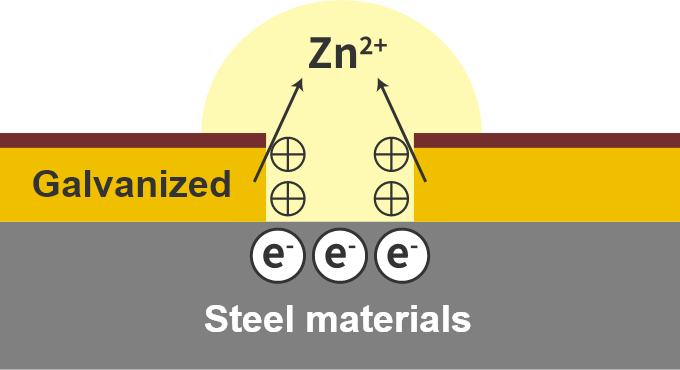
First, let me explain the principle of ionization tendency.
Ionization tendency indicates the ease of oxidation (≒ rustiness).
The greater (baser) the ionization tendency, the more easily it is oxidized.
Consider this with galvanizing.
If the galvanized coating is scratched, the steel material will be exposed and the steel may appear to rust, but in this case the galvanized coating only rusts, not the steel.
This is because the rust-prone galvanization rusts faster than the steel.
This is called sacrificial corrosion protection.
Galvanized to prevent rusting of steel
Plating pretreatment
Basic pretreatment process
-
Degreasing
- Soaking
- Vibration
- Air blow
- Electrolysis
- Ultrasonic waves
- Spray
-
Rinsing with water
-
Soak in an acidic solution
- Soaking
- Electrolysis
-
Rinsing with water
-
Electrolytic cleaning
- Anode
- Cathode
- PR
-
Rinsing with water
-
Neutralization (activation)
- Soaking
-
Rinsing with water
-
Plating
Purpose and action of each treatment process
| Type of works | Cold region | |||||
|---|---|---|---|---|---|---|
| Degreasing | Rustproofing | Descaling | Desmutting | Removing buffing shavings | Activation | |
| Degreasing |  |
 |
 |
 |
 |
 |
| Soak in an acidic solution |  |
 |
 |
 |
 |
 |
| Electrolytic cleaning |  |
 |
 |
 |
 |
 |
| Neutralization (activation) |  |
 |
 |
 |
 |
 |
Purpose and Performance (Excellent 




Purpose and action of each treatment process
-
Before cleaning
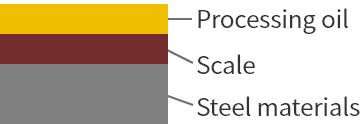
-
Degreasing

Degreasing removes most of the adhered oil.
-
Soak in an acidic solution
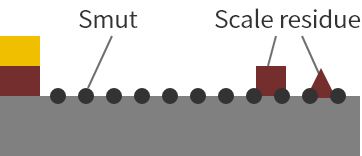
Soaking in an acid solution removes most of the scale. However, smut occurs at the same time. In poorly degreased areas, oil inhibits the action of the acid.
-
Anodic electrolytic cleaning

Acid smut generated in the acid soaking process is removed by anodic electrolysis, but the surface of the material is thinly oxidized.
-
Neutralization (activation)

Oxide film is removed and a clean surface is obtained.
However, poorly degreased areas that could not be removed in the 2. degreasing process render all subsequent processes invalid.
Chemical conversion treatment
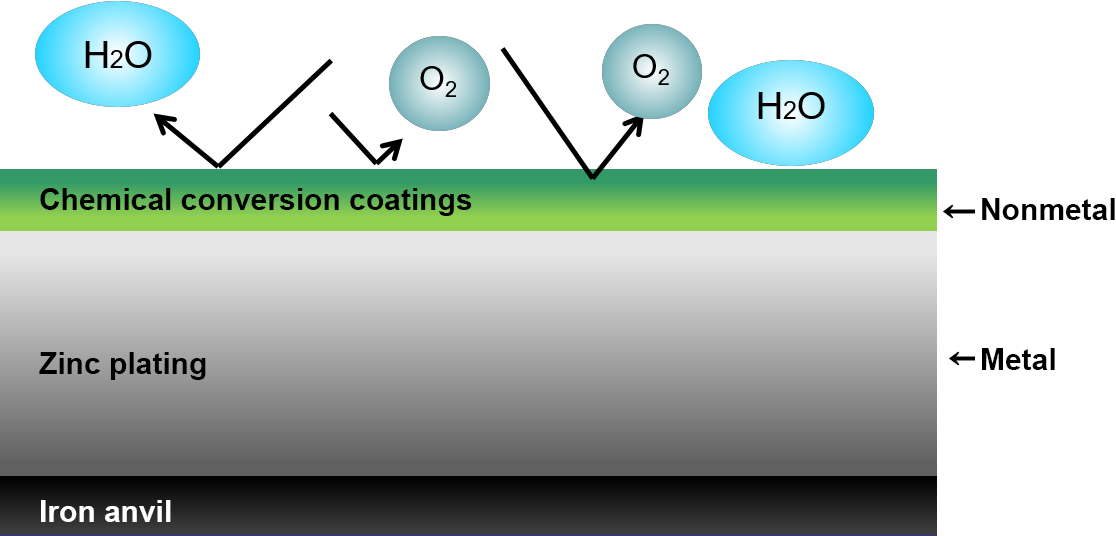
For the purpose of improving corrosion resistance, appearance, and adhesion as a base metal for painting, this is a surface treatment method that chemically coats metal surfaces by forming a film of non-metallic substances such as oxides, phosphates, and sulfides.
Looking at treatment methods, electrochemical methods include anodic oxidation, while chemical methods include chromate treatment, phosphate treatment, and chemical coloring.
Conversion treatment of zinc plating
Electrogalvanized steel sheets are rarely used in their galvanized form because zinc, a base metal, quickly loses its appearance due to the formation of white rust, a corrosion product of zinc, caused by moisture and dirt in the atmosphere.
In most cases, a trivalent chromium conversion treatment is applied as a post-treatment. For galvanized steel sheets, trivalent chromium conversion treatment not only significantly improves corrosion resistance, but also improves the appearance and properties of the plating. Currently, there are two main colors that differ in appearance: silvery-white and black.
Trivalent chromium conversion treatment
Basic pretreatment process
-
Zinc plating
-
Rinsing with water
-
Activation
-
Rinsing with water
-
Trivalent chromium conversion treatment
-
Rinsing with water
-
Finishing treatment
-
Drying
-
Activation
Immersion in 0.2 to 1% diluted nitric acid for about 5 seconds removes the plating brightener and at the same time activates the zinc surface to allow for normal conversion treatment.
-
Trivalent chromium conversion treatment
By immersing in an acidic solution based on trivalent chromium salts, a film with a chromium oxide or chromium hydroxide skeleton is formed on the galvanized surface through a chemical reaction.
-
Finishing treatment
If necessary, it is treated to improve corrosion resistance, appearance, coefficient of friction stabilization, and other functions.(May be omitted in some cases.)
Trivalent chromium conversion coatings structure and corrosion prevention mechanism
Schematic diagram of trivalent chromium conversion coatings
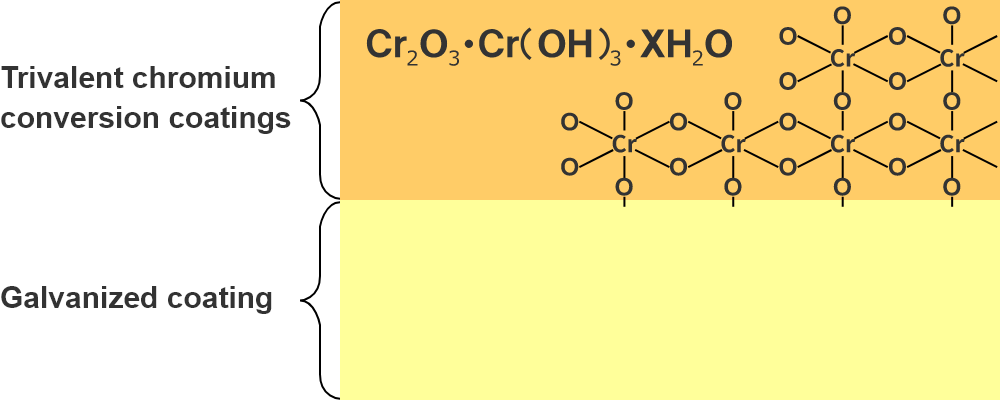
As shown in the schematic diagram, trivalent chromium conversion coatings are mainly composed of chromium and oxygen, and are three-dimensional coatings composed at the molecular level. They are very dense and provide an excellent barrier against corrosive factors (atmospheric chlorine gas, sulfurous acid gas, etc.).
In addition, this coating is in a passivated state (chemically stable), making it less susceptible to corrosion factors. These synergistic effects provide strong protection for galvanized coatings.

